Assorted Links Monday
Office politics at home, the American Academy of Pediatrics vs the FDA on the COVID vaccine for kids, the times we spend read, and more.

Table of Contents
Office politics move into the home: As the pandemic drags on — keeping millions of Americans teleworking, and countless students studying remotely — the tense dynamics once confined to the office have infiltrated people's houses and apartments.
The American Academy of Pediatrics has written a stunning letter to the FDA:
We understand that the FDA has recently worked with Pfizer and Moderna to double the number of children ages 5-11 years included in clinical trials of their COVID-19 vaccines. While we appreciate this prudent step to gather more safety data, we urge FDA to carefully consider the impact of this decision on the timeline for authorizing a vaccine for this age group. In our view, the rise of the Delta variant changes the risk-benefit analysis for authorizing vaccines in children. The FDA should strongly consider authorizing these vaccines for children ages 5-11 years based on data from the initial enrolled cohort, which are already available, while continuing to follow safety data from the expanded cohort in the post-market setting. This approach would not slow down the time to authorization of these critically needed vaccines in the 5–11-year age group.
Will remote workers get left behind in the hybrid office? The benefits of working from anywhere can also come with bias against those who aren’t seen around the hallways.
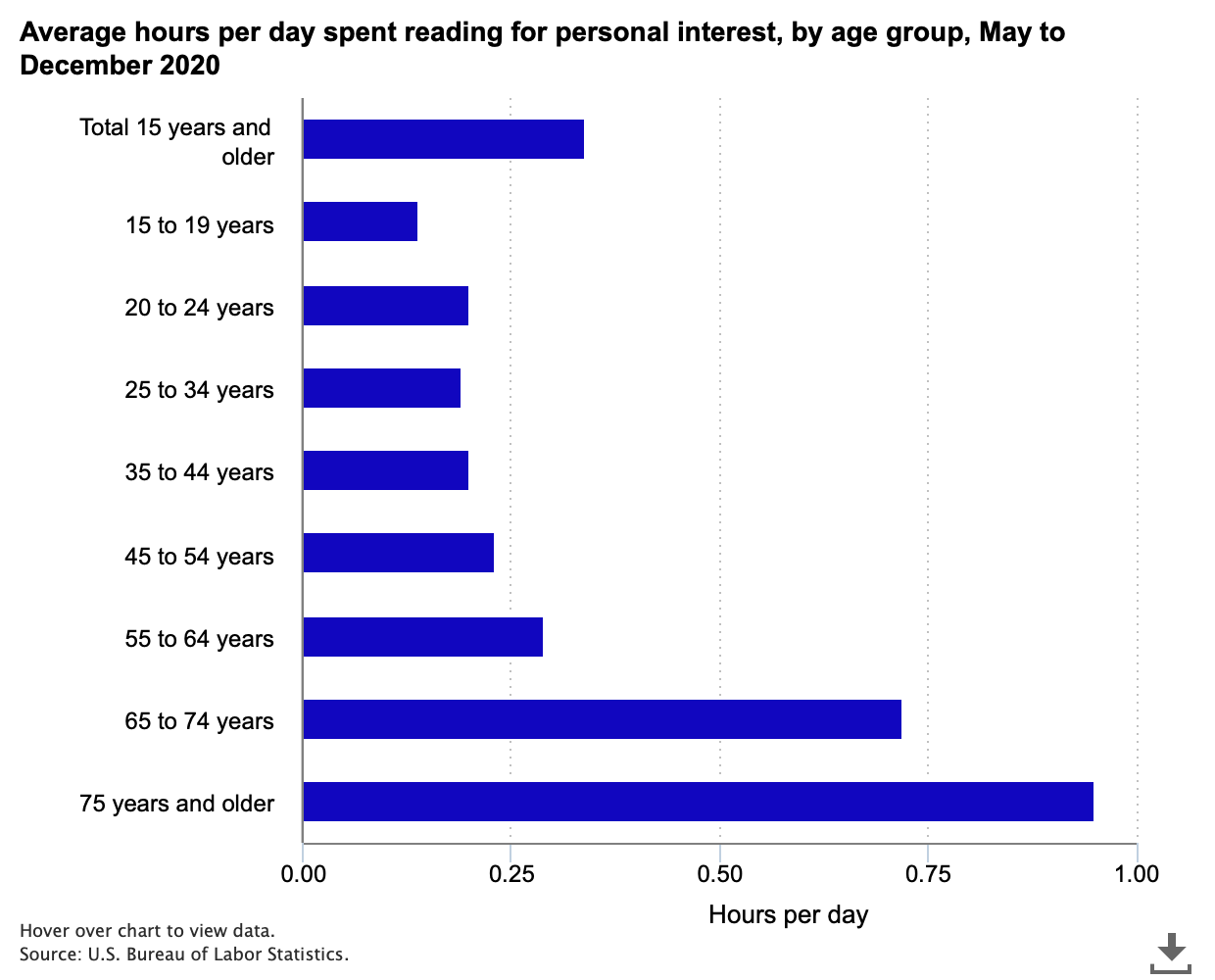
Time spent reading for personal interest in 2020
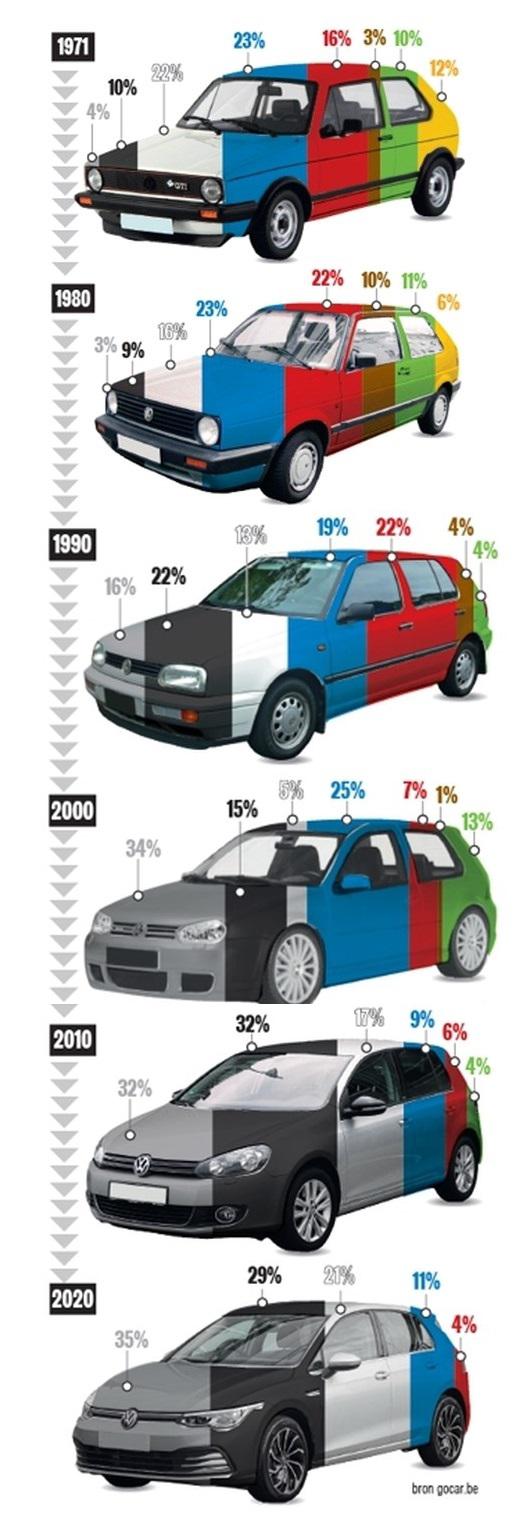
Popular car colors over time:
Econ Dev Show Newsletter
Join the newsletter to receive the latest updates in your inbox.


